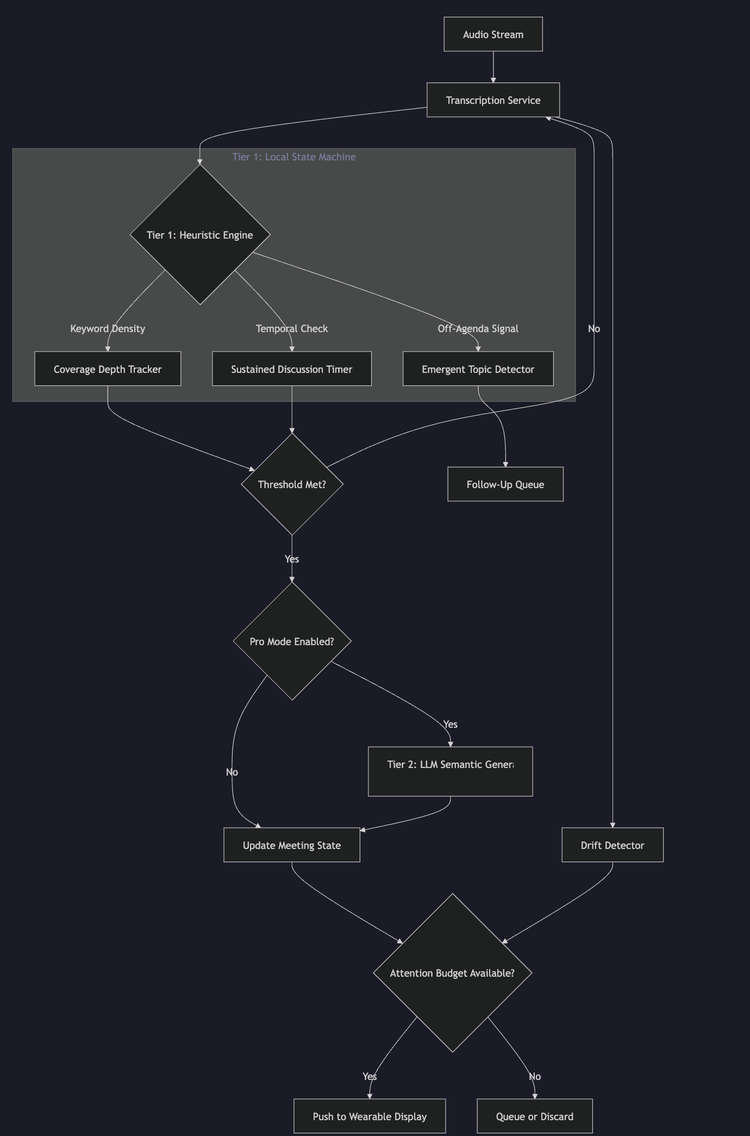
Engineering Real-Time Conversation Intelligence: A Hybrid Heuristic-Semantic Architecture for Wearable Devices
Abstract: This document discloses a novel system architecture for real-time conversation coaching on bandwidth-constrained wearable devices. The system solves the latency-intelligence trade-off inherent in LLM-based applications by implementing a tiered heuristic-semantic state machine
The Auditor Who Bet on AI: Why I Left California, Moved to Illinois, and Built Tools to Make AI Auditable
The Auditor Who Bet on AI: Why I Left California, Moved to Illinois, and Built Tools to Make AI Auditable
Your Next Top Performer Isn't in Your Talent Pipeline—They're Running a Guild
The workforce optimized for AI-augmented, distributed environments already exists. They have been training for it for decades.
While traditional organizations
GxP AI Workflow Cheat Sheets
Pilot Program: AI-Augmented Quality Engineering
1. Process Validation Assistant
Objective: To instantly locate and cross-reference key process validation documents and
Consciousness in Machines: Substrate Independence and the Hard Problem
What if a non-biological system could one day host something like experience?
Problem / Context
For decades, consciousness was reserved for
P vs NP as Physics: When Computational Limits Define Reality
What if the greatest mystery in computer science—P versus NP—isn’t just math problem, but a window into
The Universe Might Be One Giant Learning Algorithm
Imagine this: every leaf, cloud, and cell as lines of code—what if reality is a learning algorithm waiting to
The Hard Problem of AI Consciousness
Modern AI, from voice assistants to text generators, performs astonishing feats—
The Thought-Provoking Ideas and Theories from Lex Fridman and Demis Hassabis
Listening to Lex Fridman's episode 475 with Demis Hassabis, this conversation represents one of the most intellectually rich